Risk of oral antifungal agent-induced liver injury in Taiwanese
- PMID: 23750489
- PMCID: PMC3895359
- DOI: 10.1111/bcp.12178
Risk of oral antifungal agent-induced liver injury in Taiwanese
Abstract
Aim: Oral antifungal agent-induced liver injury is a common safety concern that may lead to patients' hesitation in treating fungal infections such as onychomycosis. This study evaluated risk of drug-induced liver injury (DILI) caused by oral antifungal agents in Taiwanese populations.
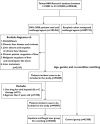
Methods: A population-based study was conducted by analyzing who used oral antifungal agents from 2002 to 2008 from the Taiwan National Health Insurance Database. A comparison control group was randomly extracted from the remainder of the original cohort.
Results: Of the 90,847 oral antifungal agents users, 52 patients had DILI. Twenty-eight DILI cases used ketoconazole, 12 fluconazole, eight griseofulvin, three itraconazole and two terbinafine. The incidence rates (IR) of DILI per 10,000 persons were 31.6, 4.9, 4.3, 3.6 and 1.6 for fluconazole, ketoconazole, griseofulvin, itraconazole and terbinafine, respectively. Longer exposure duration increased the risk of DILI, with IR for exposure duration ≥ 60 defined daily dose (DDD) of 170.9, 62.5, and 36.1 per 10,000 persons for ketoconazole, itraconazole and terbinafine, respectively. Patients taking antifungal agents had higher incidences of developing DILI compared with those in the control group after adjusting for age, gender and co-morbidities (relative risk 2.38, P < 0.001). All of the six patients with fatal DILI used fluconazole. Old age and fluconazole increased the risk of oral antifungal-induced fatal DILI.
Conclusions: Oral antifungal agents are associated with low incidence of acute liver injury, but which may be fatal, especially for the elderly. Longer treatment duration may increase the risk of antifungal agent-induced liver injury, especially ketoconazole.
Keywords: Taiwanese; drug-induced liver injury; hepatitis; oral antifungal agents.
© 2013 The British Pharmacological Society.
Figures
Similar articles
-
A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs.Br J Clin Pharmacol. 1999 Dec;48(6):847-52. doi: 10.1046/j.1365-2125.1999.00095.x. Br J Clin Pharmacol. 1999. PMID: 10594489 Free PMC article.
-
Efficacy of itraconazole, terbinafine, fluconazole, griseofulvin and ketoconazole in the treatment of Scopulariopsis brevicaulis causing onychomycosis of the toes.Dermatology. 2001;202(3):235-8. doi: 10.1159/000051643. Dermatology. 2001. PMID: 11385230 Clinical Trial.
-
A risk-benefit assessment of the newer oral antifungal agents used to treat onychomycosis.Drug Saf. 2000 Jan;22(1):33-52. doi: 10.2165/00002018-200022010-00004. Drug Saf. 2000. PMID: 10647975 Review.
-
Systemic antifungal therapy for tinea capitis in children.Cochrane Database Syst Rev. 2016 May 12;2016(5):CD004685. doi: 10.1002/14651858.CD004685.pub3. Cochrane Database Syst Rev. 2016. PMID: 27169520 Free PMC article. Review.
-
Oral Azole Antifungal Medications and Risk of Acute Liver Injury, Overall and by Chronic Liver Disease Status.Am J Med. 2016 Mar;129(3):283-91.e5. doi: 10.1016/j.amjmed.2015.10.029. Epub 2015 Nov 17. Am J Med. 2016. PMID: 26597673 Free PMC article.
Cited by
-
Hepatotoxicity Induced by Azole Antifungal Agents: A Review Study.Iran J Pharm Res. 2023 Apr 9;22(1):e130336. doi: 10.5812/ijpr-130336. eCollection 2023 Jan-Dec. Iran J Pharm Res. 2023. PMID: 38116543 Free PMC article. Review.
-
Inverse association between hepatitis B virus infection and fatty liver disease: a large-scale study in populations seeking for check-up.PLoS One. 2013 Aug 22;8(8):e72049. doi: 10.1371/journal.pone.0072049. eCollection 2013. PLoS One. 2013. PMID: 23991037 Free PMC article.
-
Adverse Effects Associated with Long-Term Administration of Azole Antifungal Agents.Drugs. 2019 Jun;79(8):833-853. doi: 10.1007/s40265-019-01127-8. Drugs. 2019. PMID: 31093949 Review.
-
Retrospective Cohort Study of Hepatic and Hematologic Toxicity in Terbinafine-Treated Onychomycosis Patients With Reduced Kidney Function at an Academic Institution.Dermatol Pract Concept. 2024 Apr 1;14(2):e2024137. doi: 10.5826/dpc.1402a137. Dermatol Pract Concept. 2024. PMID: 38810042 Free PMC article. No abstract available.
-
Acarbose Use and Liver Injury in Diabetic Patients With Severe Renal Insufficiency and Hepatic Diseases: A Propensity Score-Matched Cohort Study.Front Pharmacol. 2018 Aug 7;9:860. doi: 10.3389/fphar.2018.00860. eCollection 2018. Front Pharmacol. 2018. PMID: 30131698 Free PMC article.
References
-
- Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–485. - PubMed
-
- Bjornsson E. Review article: drug-induced liver injury in clinical practice. Aliment Pharmacol Ther. 2010;32:3–13. - PubMed
-
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. - PubMed
-
- Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. 2007;25:1135–1151. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials